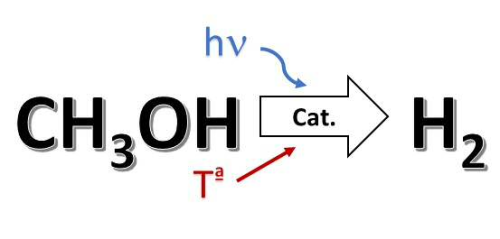
CSIC has developed a new method hydrogen production by methanol reforming using a photo-thermocatalyst, which combines thermal and light energy for the activation. The method allows to moderate the operating conditions (temperature and pressure), leading to energy savings and an improvement in the stability of the catalyst.
Industrial partners from the energetic industry interested in using this method under a patent licence agreement, are being sought.
Synergic effect of the thermal and light energy Hydrogen has multiple industrial applications, but above all, constitutes a present and future energy vector for the storage and transport of clean energy. The conversion of methanol into hydrogen is achieved through a reforming process, which requires a lot of energy, normally, high temperature and moderate pressure. For this reason, it is necessary to look for improvements of the process that allow milder reaction conditions. In this context, the multicatalytic approach to thermocatalysis may represent an interesting solution. The method of this invention consists on taking the advantage of the synergic effect of thermal and light energy (may even be solar), for the activation of the hydrogen production reaction, which implies a decrease in the reaction temperature, with the subsequent energy saving. With this photo-thermo-catalyst, several tests were carried out, achieving two effects: a decrease the temperature and an increase in the production rate of hydrogen, which can reach 50% depending on the applied conditions.
Main innovations and advantages
· The catalyst is easy to synthetize and industrially scalable · Existing reactors require minimum adaptation for the application of the two energies simultaneously.
· The method produces at least 50% more H2 with the combination of light and heat.
· The catalyst shows activity even from 120 ºC, when both energies are used.