Summary of the technology
Problem to solve:
Uveal ocular melanoma is the most common primary ocular malignancy in adults, with an incidence of approximately 7 cases per million inhabitants per year. Its incidence is therefore not very high, but it is a disease for which there are has been no alternative for more than 50 years. In addition, the American Cancer Society has published an increase in the incidence, having been diagnosed only in the United States 2,730 new cases in 2014. By the year 2018 there are anticipated 3,540 new cases in the USA.
According to www.cancer.gov, the database of the National Cancer Institute of the USA, the age-adjusted average incidence of uveal melanoma in the United States is about 4.3 new cases per million inhabitants, without a clear variation related to latitude. Men have a higher incidence than women (4.9 vs. 3.7 per million). The age-adjusted incidence of this cancer has remained stable since at least the first years of the 1970s. Incidence rates in the United States are low compared with the rates of other reporting countries, which range from 5.3 to 10.9 cases per million. Part of the variation may be the result of differences in the inclusion criteria and calculation methods.
Innovation:
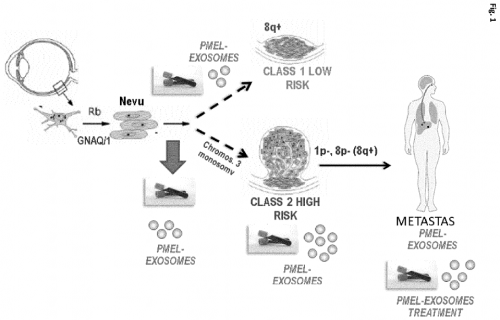
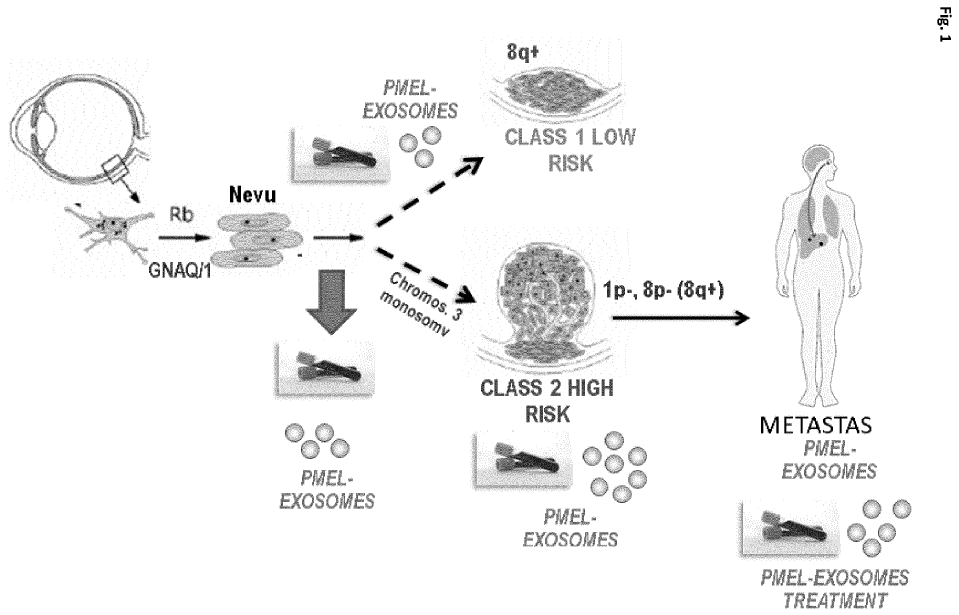
The present invention provides an in vitro method for detecting tumor growth and diagnosing or prognosticating the risk of metastasis in a human subject that has been diagnosed with uveal melanoma, wherein the biological sample is treated with a lysis agent capable of disrupting the membrane of the exosomes prior to determining the expression of the melanocyte protein PMEL/ME20-S/gp 100.
This method incorporates new techniques that differentiate it from the potential competitors in several
aspects. Our method is non-invasive (as it does not require a tumor biopsy), it does not require specific training and
sampling is included in the hospital routine (blood extraction). Besides, it can be used after the application of the treatment,
allowing not only to monitor the tumor advance and the early development of metastasis but also to help monitoring the
effect of the treatment. In addition, it would be a more economical method.
Current development status
Experimental technologies
Analytical validation already performed
Applications
Prognosis
Treatment monitoring
Desired business relationship
Patent licensing
Technology co-development
Intellectual property status
Attached documents
Related Keywords