CSIC and the University of Zaragoza have developed a new formulation to achieve a controlled and sustained release of different drugs inside the eyeball.
Industrial partners from the ophthalmic or pharmaceutical industry are being sought to collaborate through a patent licence agreement.
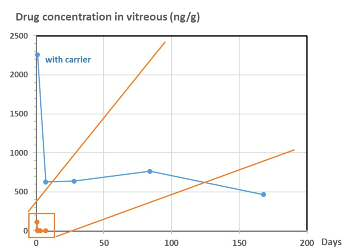
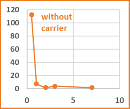
Inorganic carrier for controlled release of ophthalmic drugs Intravitreal injections are invasive and the associated risks (retinal detachments, opportunistic infections, cataracts) increase when repeated applications are required. The short half-life of substances (drugs) administered via this route requires frequent injections for the maintenance of effective concentrations, in the treatment of chronic eye diseases, with poor patient compliance. In base of the short half-life of the drugs, alternative drug delivery systems and sustained-release inserts are being developed to overcome this limitation by reducing the frequency of injections. Therefore, there is still a need to administering drugs, in a controlled released manner, to the posterior segment of the eye for the treatment of retinal and choroidal diseases. Drug concentration in vitreous (ng/g)
Main innovations and advantages
· The main advantage of the present technology is the very significant reduction of the injections frequency.
· The formulation comes in powder form, but is injected as a transparent colloidal dispersion, making it ideal for intravitreal administration without interfering with the vision.