CSIC has developed a chimeric protein that, when expressed in bacteria, changes its structure upon
absorption of blue light, thus generating protein aggregates (amyloids) that inhibit bacterial growth
Industrial partners from the pharmaceutical industry are being sought to collaborate through a patent
licence agreement
An offer for Patent Licensing
A new, sustainable and clean way to inhibit bacterial growth
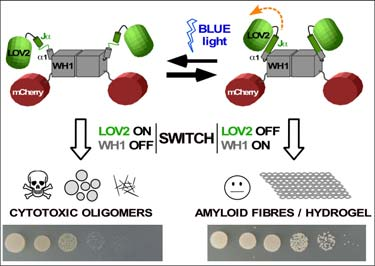
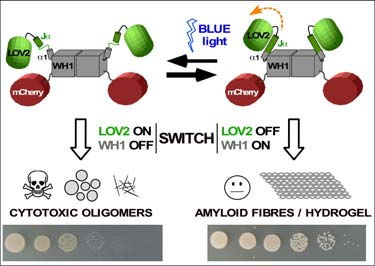
Optogenetic device to control amyloid assembly and bacterial growth (‘optobiotic’). Blue light illumination of a photosensor LOV2 domain (green), fused to WH1 (grey) and to a fluorescent marker (red), promotes the formation of WH1 amyloid oligomers that inhibit bacterial growth (bottom: spot assay with E. coli) In this patent, the power of optogenetics to engineer proteins as light- responsive switches has been used to control the balance between solubility and amyloid aggregation for LOV2-WH1, a chimera between the plant blue light-responsive domain LOV2 and the bacterial prion-like protein RepA- WH1.
In the darkness and in vitro, LOV2-WH1 nucleates the irreversible assembly of amyloid fibres into a hydrogel. However, under blue light illumination LOV2-WH1 assembles as soluble oligomers.
When expressed in the bacterium Escherichia coli, LOV2-WH1 forms in the darkness large intracellular amyloid inclusions compatible with bacterial proliferation. Strikingly, under blue light LOV2-WH1 aggregates decrease in size while they become detrimental for bacterial growth.
LOV2-WH1 optogenetics governs the assembly of mutually exclusive inert amyloid fibres or cytotoxic oligomers, thus enabling the navigation of the conformational landscape of protein amyloidogenesis to generate a new kind of photo-activated anti-bacterial devices (optobiotics).
Main innovations and advantages
· A synthetic protein construction (LOV2-WH1) is activated as an anti-microbial by a physical, harmless stimulus: blue light · Blue light absorption by LOV2-WH1 stimulates the assembly of anti-bacterial protein particles whereas, in the darkness, these are inert · Mobilization of LOV2-WH1 through vectors/bacteriophages wouldenable its usage against a broad spectrum of bacterial pathogens · LOV2-WH1 is amenable to photo-therapy procedures aiming tocombat bacterial skin infections, or to de-contaminate pathogens fromsurfaces · In mixed bacterial populations assembled in an industrial bioprocess,optogenetic activation of LOV2-WH1 can be used to selectivelyeliminate a subpopulation, e.g. once achieved its intended task