The present data provide a solid basis for the development of genetic biomarkers for the early identification of patients suffering from dementia with Lewy bodies and to classify them into specific molecular subtypes. One of the biomarkers correlates with the development of Lewy pathology in the brain and may be useful to monitor the success of possible alpha-synuclein antiaggregatory therapies.
Background
Dementia with Lewy bodies (DLB) belongs together with Parkinson’s disease (PD) to the group of Lewy body diseases and is after Alzheimer disease (AD) the second cause of dementia. Within this spectrum DLB shows overlapping features with both above mentioned conditions making difficult its correct diagnosis. Nevertheless, DLB is characterized by an aggressive disease course and overall elevated mortality turning an early and accurate diagnosis to a need. Similar to AD and PD, DLB is very heterogeneous and various subgroups develop disease by their own molecular mechanisms each. Therefore, it is hard to suppose that only one biomarker will identify all DLB patient. Instead, different biomarkers, one for each of the various subgroups, will be needed to identify all DLB patients. Only working strategies in this line further permit to tackle the challenges of personalized medicine.
Our results up to day
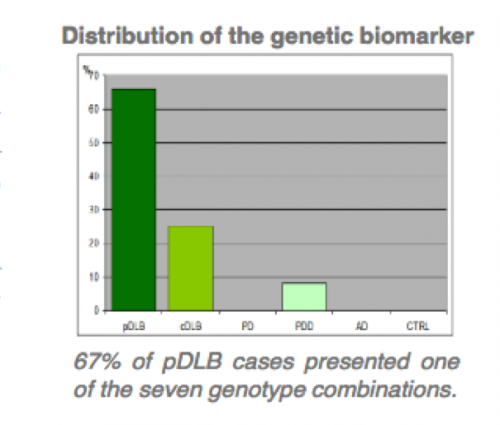
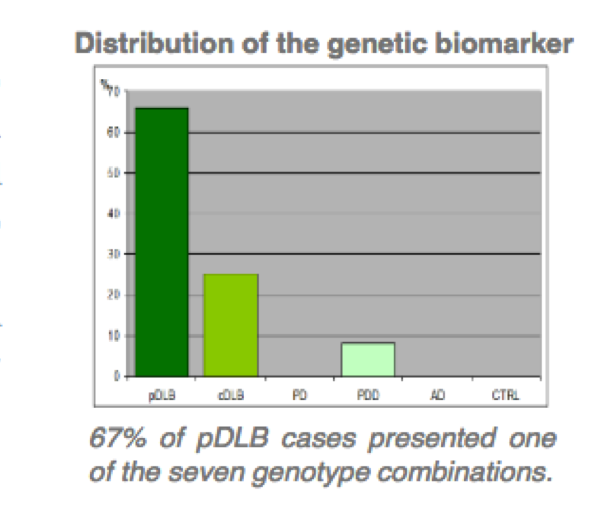
The technology provides a different in vitro methods for the diagnosis of dementia with Lewy (DBL) bodies and DBL subgroups related with two gens. –SNCA, SNCB divided in two patent assets.
- Method for the diagnosis of pure DBL by PCR techniques, related with presence of four gene SNCB variations.
- Specificity above 95%.
- Comercial use as PCR kit for companion diagnostics to treat with distinctive medical regime DBL patients.
- Method for the diagnosis of DBL, based in two SNCA transcripts SNCAtv2 and SNCAtv3 in blood samples.
- Sensitivity and specifity of transcripts for the diagnosis of DLB were above 90%.
- Comercial use as kit for the main biotecnological procedures: PCR, QPCR, multiplex PCR, NASBA, LCR, RT-PCR, RNA sequencing, Northern.
Advantages
•The use of these genetic biomarkers will permit to identify those patients with DLB that belong to subgroups with known molecular changes in the brain.
• The potential to personalize the diagnosis and possibly also treatment is becoming a promising strategy for clinical use.
• One of the biomarkers will allow to monitor the success of therapies to be applied in patients with dementia.
• The facility of standardization and relative low-cost of analysis are key characteristics for the use of the biomarker in clinics.
Intellectual property status
PCT application
Desired business relationship
• Co-development
• Licensing Out
Diagnostic Biomarker for dementia with lewy bodies