Never miss an update from Universidad de Alicante
Create your free account to connect with Universidad de Alicante and thousands of other innovative organizations and professionals worldwide
The Materiales Carbonosos y Medio Ambiente (MCMA) research group at the University of Alicante has developed a new procedure to prepare heterogeneous catalysts from biomass waste based on highly dispersed metal nanoparticles.This procedure is characterised by the fact that it is very simple, involves few synthesis steps, the synthesis conditions are mild and it is environmentally friendly. Moreover, it is easy to scale up to an industrial level, allows the revalorisation of any type of biomass-derived waste and has a low manufacturing cost. The synthesised catalysts show excellent catalytic behaviour using low noble transition metal contents and could become very promising candidates to replace current commercial catalysts in the production of molecules of great interest to the chemical sector, such as, for example, in the conversion of levulinic acid to gamma-valerolactone. Companies interested in acquiring this technology for commercial exploitation are sought
TECHNICAL DESCRIPTION
In order to solve the problems described above, a new process has been developed to prepare heterogeneous catalysts consisting of carbonaceous materials derived from biomass residues and highly dispersed metal nanoparticles with low transition metal content.
The process to obtain these novel catalysts comprises the following steps:
1. Process the biomass. Biomass waste rich in lignocellulose (it can be any type of biomass, for example: cocoa shells, almond shells, hemp, eucalyptus, etc.) is subjected to a grinding and sieving process to achieve an optimum particle size. Subsequently, they are washed to remove inorganic matter. Finally, they are dried in an oven.
2. Carbonising the processed biomass residues in an autoclave reactor in the presence of an aqueous solution (this process is called hydrothermal carbonisation). The heat treatment is carried out at a moderate temperature for a certain time.
3. Activating the carbonised product obtained in the previous stage. The carbonised product is subjected to an activation heat treatment in a tube furnace using a specific heating ramp until a certain temperature is reached, which is maintained for a certain period of time. This process is carried out in an inert atmosphere.
4. Washing the activated carbon resulting from the previous stage. For this purpose, different washes are carried out with distilled water at moderate temperature until a neutral pH is reached.
5. Dry the activated carbon. The activated carbon obtained in the previous step is dried at a certain temperature for a specific time.
6. Impregnate the activated carbon with the metal precursor. To an aqueous dispersion of activated carbon, an aqueous solution of an inorganic salt of a transition metal (ruthenium, palladium, iron or rhenium) is added and stirred at room temperature for a specified time.
7. Reduce the metal phase with a reducing agent. To the above suspension, an aqueous solution of a reducing agent (preferably a metal hydride) is added at a given concentration and stirred at room temperature for a specified time. Subsequently, the catalyst obtained is filtered and washed with distilled water to remove the solvent.
8. Dry the obtained heterogeneous catalyst. For this purpose, a moderate temperature is used for a specified time.
TECHNOLOGY ADVANTAGES AND INNOVATIVE ASPECTS
ADVANTAGES OF THE TECHNOLOGY
The main advantages of this novel procedure are listed below:
1) It comprises few steps and is very simple.
2) It is carried out under mild reaction conditions: low pressure, moderate temperature and short reaction times.
3) It avoids the use of hydrogen gas at high temperatures.
4) The formation of small metal nanoparticles is favoured.
5) High dispersion of the metal nanoparticles on the carbonaceous supports is achieved.
6) No aggregates of metal particles are obtained.
7) The catalyst offers many active sites for the chemical reaction in which it is to be used, which gives better results than with commercial catalysts.
8) Drying of the catalyst is carried out at a lower temperature than conventional methods, which prevents the electronic properties of the surface of the metal nanoparticles from changing substantially.
9) It allows the recovery of abundant biomass waste (cocoa shells, almond shells, hemp, eucalyptus, etc.).
10) The catalysts obtained can be used in the chemical conversion of a multitude of molecules of great industrial interest.
11) Lower production costs than current synthesis methods.
12) Lower environmental impact than current synthesis methods.
13) Porosity is achieved equal to or higher than with the conventional activation process.
14) Higher yields are achieved compared to conventional chemical activation.
15) The process is easily scalable to industrial scale.
16) Versatility of the synthesis method: different types of hard or soft lignocellulosic biomass residues can be used (regardless of their composition and moisture content).
17) Low metal content (ruthenium, etc.) compared to commercial catalysts.
18) Higher levulinic acid conversions (98.4%) and selectivities towards GVL (100%) are achieved than in the current state of the art.
19) The catalysts show excellent catalytic activity under mild reaction conditions (low temperatures, etc.).
20) High stability of the catalysts obtained after several consecutive reaction cycles.
21) No special equipment is required: the equipment used is commercially available and affordable for any laboratory or industry.
22) The precursors used are very cheap and abundant.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
The main innovation lies in the use of agricultural waste (lignocellulosic biomass) to obtain heterogeneous catalysts containing low concentrations of ruthenium in the form of highly dispersed metal nanoparticles.
The present invention differs from current synthesis methods in that:
1) Activated carbons obtained from biomass residues rich in lignocellulose are used as support for the active phase.
2) Ruthenium contents are much lower than those present in commercial catalysts.
3) Mild reaction conditions are used.
4) The low temperature used for catalyst drying prevents the electronic properties of the surface of the metal nanoparticles from changing substantially.
5) The metal nanoparticles are highly dispersed on the surface of the activated carbon support, allowing many active sites for the chemical reaction of interest to take place with high efficiency and selectivity.
6) The activating agents used are not dangerous for the environment and, moreover, very low concentrations are used compared to conventional chemical activation, which reduces synthesis costs and environmental impact.
7) The method is very simple, with few steps and short synthesis times.
CURRENT STATE OF DEVELOPMENT
These novel heterogeneous catalysts (see Picture 1) have been successfully synthesised at laboratory level. This technology is at a stage of maturity TRL = 4 (Technological Readiness Level).

Picture 1: Synthesised catalyst in fine powder form.
The heterogeneous catalysts obtained by this novel process are characterised by the following features:
• They have surface areas between 700-2.000 m2·g-1.
• The active metal phase is highly dispersed in the form of nanoparticles with an average size between 1.6-3.0 nm.
• The final transition metal content is between 0.1-0.60% weight.
• In the different reaction conditions tested, the catalytic activity in the hydrogenation of levulinic acid to GVL has values very close to 100%.
Some examples of different biomass residues used in the laboratory tests to synthesise these novel catalysts are shown below (see Picture 2):
Picture 2: Different biomass residues used in the laboratory tests, including cocoa shells, eucalyptus wood and almond shells, respectively.
The catalysts obtained have been characterised using various techniques to determine their structure and composition, among them:
• N2 adsorption isotherms to determine the porous texture.
• Transmission Electron Microscopy (TEM) to determine the morphology of the active metal phase and the average size of the nanoparticles (see Figure 3).
• Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) to determine the final transition metal content.
• X-ray Photoelectron Spectroscopy (XPS) to determine the different transition metal species and their surface content.
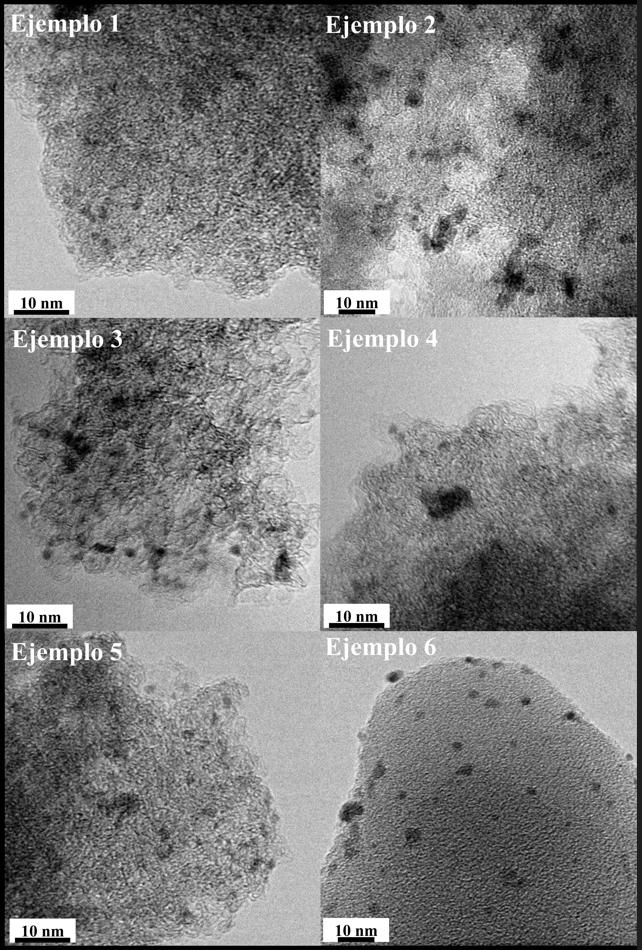
Picture 3: TEM micrographs of the different catalysts synthesised.
COLLABORATION SOUGHT
Companies interested in acquiring this technology for commercial exploitation are sought:
• Patent licensing agreements.
• Development of new applications.
• Technology and knowledge transfer agreements.
Company profile sought:
• Catalyst manufacturers.
Ahead of the current Coronavirus outbreak, Innoget is fully committed to contributing to mobilizing scientific and expert communities to find a real solution to the Covid-19 pandemic. Therefore, we're supporting worldwide calls and programs that could help in any aspects of the coronavirus crisis.
Is your organization promoting or looking for innovation or research initiatives to mitigate the Covid-19 outbreak? Email us at covid19@innoget.com to list them.
Channeled through Innoget's online open innovation network, initiatives in the health, virology, medicine, or novel technologies applied to human health, among others, are listed and disseminated to Innoget members -ranging from hospitals, research institutes, scientists, businesses, and public administrations- and innovation partners worldwide.
Create your free account to connect with Universidad de Alicante and thousands of other innovative organizations and professionals worldwide
Send a request for information
to Universidad de Alicante
Technology Offers on Innoget are directly posted
and managed by its members as well as evaluation of requests for information. Innoget is the trusted open innovation and science network aimed at directly connect industry needs with professionals online.
Need help requesting additional information or have questions regarding this Technology Offer?
Contact Innoget support