Never miss an update from Universidad de Alicante
Create your free account to connect with Universidad de Alicante and thousands of other innovative organizations and professionals worldwide
The Photochemistry and Electrochemistry of Semiconductors research group at the University of Alicante has extensive experience in the preparation of materials for use as both cathodes and anodes in a rechargeable sodium ion (Na+) and sodium metal (Na+) battery. In this line, the research group has also developed expertise in the preparation of electrolytes based on both organic and inorganic solvents. The ultimate goal is the development of both mobile and stationary batteries, with special emphasis on the latter.
TECHNICAL DESCRIPTION
In recent years, organic materials have received great attention from the scientific community as they can be both economical and environmentally friendly compounds. In the literature, studies are found on carbonyl compounds, anhydrides, carboxylates and imines that can carry out multi-electronic reactions, which are characterised by a high theoretical capacity. However, the problem with these compounds is that they are highly soluble in the electrolyte, which causes a rapid decay of the electrode capacity, limiting the cyclability of the device. Consequently, there is still a need to develop a rechargeable Na metal battery where the Na metal is stable enough in the electrolyte not to create any kind of passive layer on the surface, and where the cathode is stable enough not to dissolve in the electrolyte during charge and discharge cycles.
The problem of Na metal disappears with the use of sodium ion batteries in which sodium is intercalated in both the positive and negative electrodes. However, the energy and power density are reduced and the economic cost increased compared to the Na metal battery. The positive electrodes mentioned above can be used, as well as electrodes in which the reduction or reductive intercalation of sodium takes place (metals such as Sb, oxides such as TiO2, etc.).
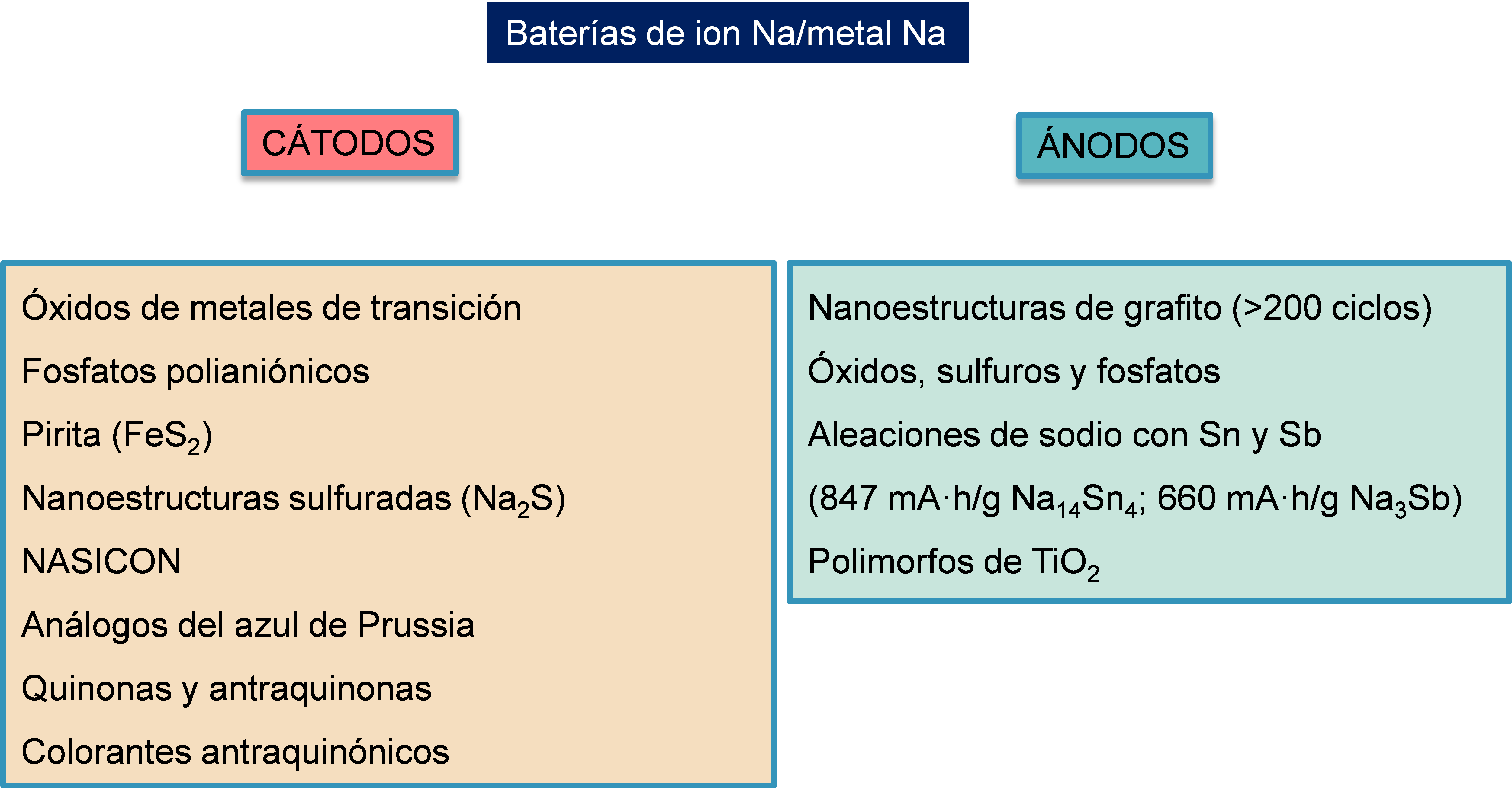
Scheme 1. Representative scheme of the main compounds used in the context of Na metal-ion batteries.
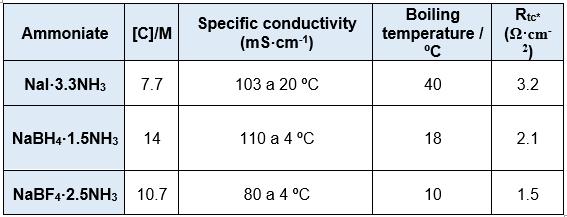
The Photochemistry and Electrochemistry of Semiconductors research group at the University of Alicante has studied a series of inorganic liquids based on ammonia or liquid ammonates whose formulation is given by NaY·xNH3, where Y corresponds to the sodium anion, which in this case can be I-, BF4- and BH4-and x represents the number of ammonia molecules per sodium ion. These liquids are characterised by a high concentration of Na+ ions above 7 M and a high ionic conductivity above 100 mS·cm-1. They have the particularity of being very low in viscosity and, in addition, sodium metal is highly stable in them. They are very cost-effective because they are based on ammonia and common inorganic salts. An additional advantage of these electrolytes is that the Na+ + 1e- → Na process shows a coulombic efficiency very close to 100% for more than 500 cycles, with no evidence of dendrite formation or SEI on the Na surface. The Na electrodeposition process can be successfully carried out using a Cu foil as a collector substrate without changing the above-mentioned benefits. This offers the possibility to assemble the device in an unloaded state, thus avoiding the use of metallic Na. Figure 1 shows the electrochemical behaviour for the Na+/Na process on a Cu foil (on which a thin layer of Na metal has been previously electrodeposited). A table with the physical properties of the ammonates described is shown below.
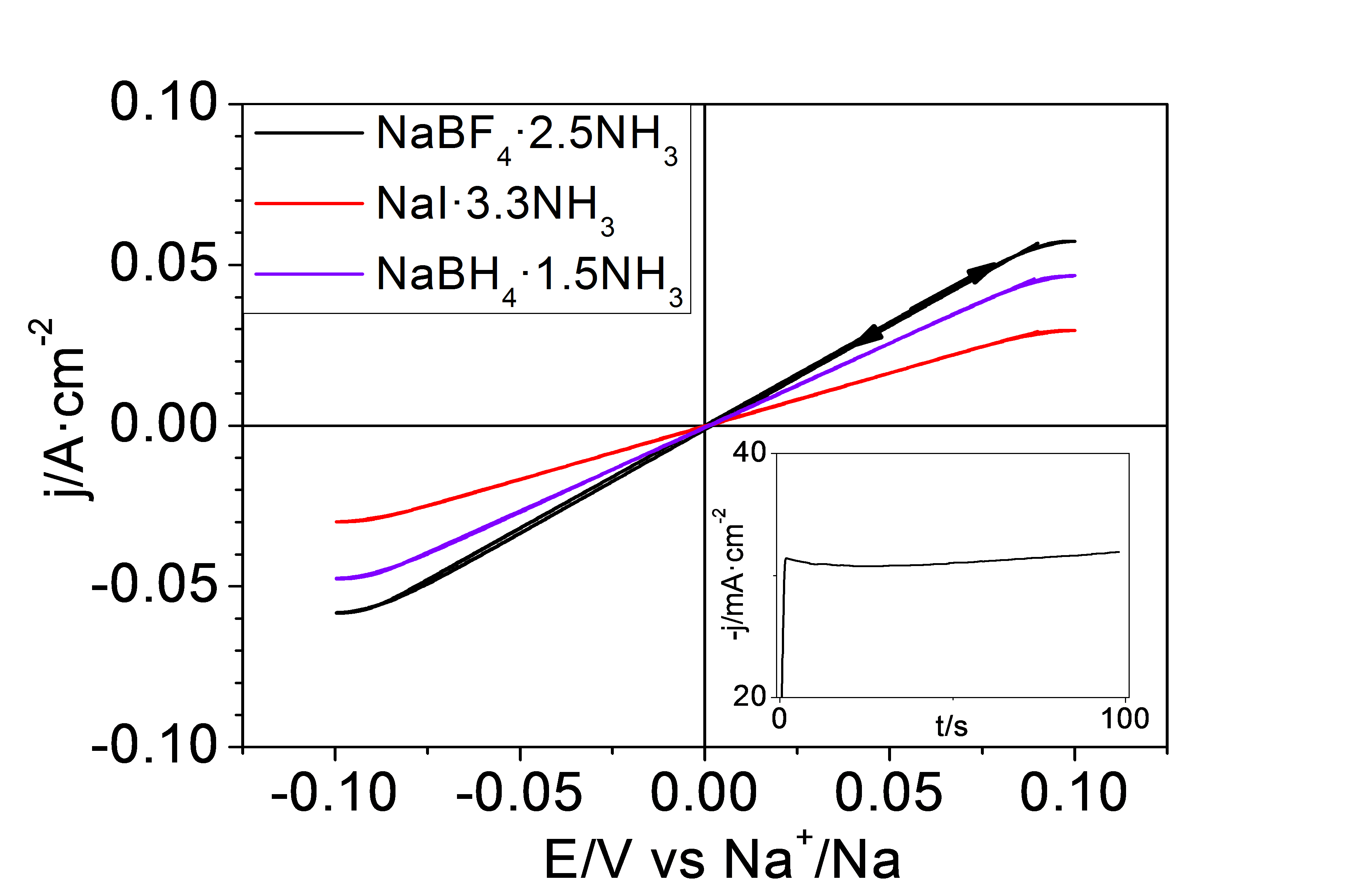
Figure 1. Cyclic voltagram at 20 mV-s-1 for the Na+/Na process on a Cu film in three inorganic electrolytes based on liquid ammonia.
Table 1. Physical properties of electrolytes based on liquid ammonia:
On the other hand, the research group has extensive experience in the electrodeposition of Na on Cu and other metals, using conventional organic electrolytes with the addition of additives capable of modifying the interface between the collector substrate and the electrolyte, in such a way that a homogeneous Na film can be deposited, free of dendrites and with a high coulombic efficiency. The most commonly used organic electrolytes are:
• 1 M NaClO4 in propylene carbonate.
• 1-3 M NaCF3SO3 in dimethoxyethane.
• 2 M NaSCN in a 1:1 v/v mixture of dimethoxyethane and 1,4-Dioxolane.
• 1 NaFSI in a 1:1 v/v mixture of dimethoxyethane and 1,4-dioxolane.
• 1 M NaTFSI in a 1:1 v/v mixture of dimethoxyethane and 1,4-dioxolane.
For the synthesis of cathode materials and the production of working electrodes, the research group has perfected various techniques depending on the nature of the active material. For the synthesis of organic materials, it requires the control of techniques involving the polymerisation of compounds, condensation reactions, addition and substitution reactions, mainly. As far as the synthesis of inorganic materials is concerned, the main focus is on the generation of the active material in a chemical bath, precipitation/encapsulation of the active material, anodising technique, etc.
As is well known, the electrodes of a battery usually consist of three elements: active material, conductive additive and binder. The research group has extensive experience in the elaboration of a homogeneous mixture between the three components of the cathode, for which techniques have been developed ranging from particle mixing in a planetary ball mill under different mixing conditions in speed and time, to particle dispersion in an aqueous or organic solvent. In addition, the ability to carry out self-anchoring reactions of the active material to the conductive additive, involving sonication and solvothermal treatments of the particles, has also been developed.
Then, once an intimate mixture of particles is available, the working electrode itself is produced. For this, the research group has used different metals as collector substrates, including:
• Nickel (Ni).
• Copper (Cu).
• Stainless steel.
• Carbon-coated aluminium (Al) foil with various coating thicknesses.
• Graphite foil.
Experience is available in the application of the mixture containing the active material by means of the techniques:
• Doctor blading.
• Drop casting.
• Dry pressing.
• Spin-coating.
• Chemical bath.
• Anodising.
It should be noted that in order to successfully carry out the preparation of all the compounds described above, from electrolytes to working electrodes, the research group has developed great skill in working in controlled atmosphere systems, either in a glove box or outside it, and in low temperature systems, for which an acetone and liquid nitrogen bath is required.
The group has been working with this type of material for several years and is therefore able to characterise it in detail in terms of its structure, composition and electrochemical properties. Electrochemical techniques for the characterisation of the materials include:
• Cyclic voltammetry.
• Galvanostatic and potentiostatic chronopotentiometry.
• Amperometric jumps.
• Electrochemical impedance spectroscopy.
As regards spectroscopic (UV-Vis, Infrared, Infrared, Raman, etc.) and microscopic techniques (scanning electron microscopy SEM, transmission electron microscopy SEM, EDX, etc.), all of them are available at the Technical Research Services of the University of Alicante.

Figure 2. Transmission electron microscope (JEOL, model JEM-2010) of the Technical Research Services of the University of Alicante.
TECHNOLOGY ADVANTAGES AND INNOVATIVE ASPECTS
ADVANTAGES OF THE TECHNOLOGY
The main advantages offered by this technology are:
1) High coulombic efficiency for the deposition and dissolution process of Na in inorganic electrolytes described as ammonates.
2) High chemical stability of the metallic Na in the ammonates.
3) High coulombic efficiency for the process of deposition and dissolution of Na in inorganic electrolytes on a Cu substrate, which offers the possibility of assembling the device in an unloaded state, thus avoiding the use of metallic sodium.
4) Work with highly concentrated inorganic electrolytes, which avoids limitations due to the transport of matter in the devices and facilitates the reversibility and homogeneity of the metal deposit.
5) Design of a sodium ion and sodium metal battery at room temperature.
6) Deposition and dissolution of Na with high coulombic efficiency in conventional organic electrolytes with additives.
7) Cost-competitive developments avoiding the use of expensive solvents, electrolytes and materials.
INNOVATIVE ASPECTS OF THE TECHNOLOGY
The first innovative aspect of the presented technology focuses on the very high stability of sodium metal in the studied inorganic electrolytes both chemically and electrochemically. These are quasi-ionic liquids consisting of simple sodium salts and ammonia. The low cost of the electrolyte, together with the possibility of working with metallic sodium, should allow the development of batteries with:
• High energy density.
• Very high response speed.
• Very low cost.
Another innovative aspect focuses on the use of organic molecular or polymeric materials with quinonic groups with the capacity to sodiumate easily. These organic electrodes are a viable alternative to electrodes based on oxides and sulphides.
CURRENT STATE OF DEVELOPMENT
The technology has been developed on a laboratory scale.
COLLABORATION SOUGHT
The research group is looking for companies/organisations to:
• Provide technical reports and scientific advice to the company.
• Offer specific training in subjects related to the synthesis and/or characterisation of electrodes, and the development and characterisation of devices.
• Training of scientific and technical personnel through the organisation of specialisation courses, seminars, technical conferences, etc.
• Offering technological support in those techniques that require a high level of training or sophisticated instruments that are not within the reach of the applicant company.
• Exchange of personnel for defined periods of time (for learning a technique, setting up a process, etc.).
• Establishing R&D&I projects with research organisations (public or private), with the aim of opening up new lines of research.
Ahead of the current Coronavirus outbreak, Innoget is fully committed to contributing to mobilizing scientific and expert communities to find a real solution to the Covid-19 pandemic. Therefore, we're supporting worldwide calls and programs that could help in any aspects of the coronavirus crisis.
Is your organization promoting or looking for innovation or research initiatives to mitigate the Covid-19 outbreak? Email us at covid19@innoget.com to list them.
Channeled through Innoget's online open innovation network, initiatives in the health, virology, medicine, or novel technologies applied to human health, among others, are listed and disseminated to Innoget members -ranging from hospitals, research institutes, scientists, businesses, and public administrations- and innovation partners worldwide.
Create your free account to connect with Universidad de Alicante and thousands of other innovative organizations and professionals worldwide
Send a request for information
to Universidad de Alicante
Technology Offers on Innoget are directly posted
and managed by its members as well as evaluation of requests for information. Innoget is the trusted open innovation and science network aimed at directly connect industry needs with professionals online.
Need help requesting additional information or have questions regarding this Technology Offer?
Contact Innoget support