Showing 1 to 15 of 2391 results


US9070809 (B2) - Inter-Facing Solar Panels
Patents for licensing Syncoda Technologies

Suppression of Impulsive Noise for 4G cell phones
Patents for licensing University of Vigo

Manganese-Nitride based Novel Magnetic Materials
Innovative Products and Technologies Georgetown University

BTS-Foam and Sediments Removal
Innovative Products and Technologies Biogas & Gases Technologies

Morphing surfaces made of slidable rods
Patents for licensing Universitat Politècnica de Catalunya - UPC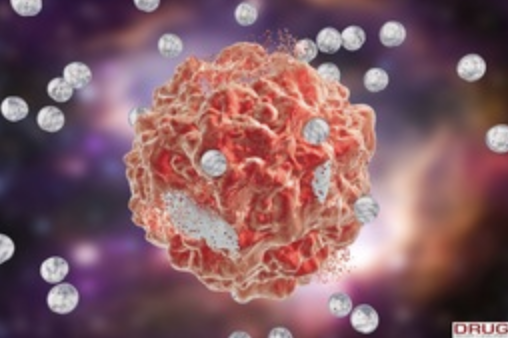

Biodegradable CCK-receptor Targeted Nanoparticle for the Treatment of Cancer
Innovative Products and Technologies Georgetown University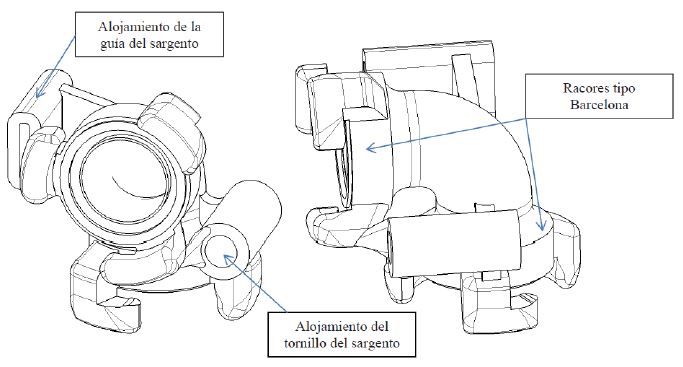

Fire hose element to facilitate the work of firefighters
Patents for licensing UNIVERSIDAD DE BURGOS

Immobilized biocatalysts for debittering citrus juices
Research Services and Capabilities UNIVERSIDAD DE BURGOS

Automatic component disassembling for dismantling and recycling systems.
Patents for licensing Universidad de Alicante

GRAPE.IO - Integrated Secure video, voice and chat Messaging
Innovative Products and Technologies EIT Digital

FN NANO®️ technology and its contribution to ESG (Environmental, Social, Governance).
Innovative Products and Technologies FN NANO CANADA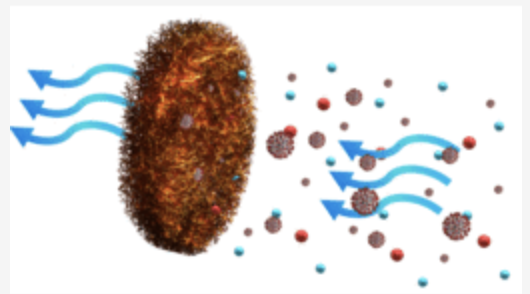

Advanced Nanoporous Metal Foams for Efficient Submicron Filtration
Innovative Products and Technologies Georgetown University

Sustainable and cost-effective method for the isolation and concentration of viruses
Innovative Products and Technologies UNIVERSIDAD DE BURGOS

Multilevel Active-Clamped Electrical Energy Converter
Patents for licensing Universitat Politècnica de Catalunya - UPC

